Longevity FAQ: A beginner's guide to longevity research
Hi! I'm Laura Deming, and I run Longevity Fund. I spend a lot of time thinking about what could increase healthy human lifespan. This is my overview of the field for beginners. Feel free to send me any questions about the below (just include name and affiliation).
Overview:
- Introduction:
- Basic concepts in aging research
- Explanation:
- Major areas of aging research
- Data:
- Comprehensive surveys of longevity
Introduction
As you get older, the chance that you will die goes up.
As you get older, the chance that you will die from certain diseases also goes up.
Why does this happen?
A simple explanation would be that, like an old car, you accumulate damage in a random fashion.
However, there are many simple things that we can do to make animals live longer. Why? We don’t really know.
Eating less makes mice live longer.
Some genes, when mutated, make mice live longer,
A few drugs, approved for human use, also make mice live longer.
There are others - we will cover them below.
So what is the study of aging?
I sum it up as the following: trying to figure out what kinds of damage accumulate with age, how to reverse that accumulation, and the search for switches that we could flip in human biology to increase lifespan.
Research areas in longevity
Caloric Restriction
at a glance: eating less, in a variety of ways, can make you live longer - but is your body just using number of calories as a signal?
In the 1930s, investigators wanted to do an experiment to see if stunted growth rates during the Great Depression might impact lifespan. They tested this in rats by feeding them less food than they would normally eat. To their surprise, this actually made the rats live longer! This was a seminal discovery. For the first time, we changed the environment of an animal to make it live longer than it normally would.
Since then, investigators have tried to uncover how this works. The effect depends on what genes you have, what you are eating and how much less you eat. If you take many genetically distinct mouse strains and put them on the same diet (cutting calories by ~40%), sometimes fewer than 1/5 of the mouse strains live longer. Diet composition also plays a role. Just decreasing protein or a specific amino acid, while keeping total calorie intake the same, can result in a lifespan extension in mice. Feeding mice a ketogenic diet also seems to help. Decreasing food intake by too much will result in starvation, so finding a diet that works can depend on the situation.
While long-term human studies are sparse, investigators have run two caloric restriction experiments in monkeys, one of which showed promising results for an increase in survival. To avoid the difficulty of continuous dieting, fasting ~8+ hours a day, or 5 days/month, or on a variety of different cycles might also be helpful. This is called intermittent fasting. Medically, intermittent fasting may aid recovery during chemotherapy. Some longevity-related pathways involve sensing amino acid levels, so it is possible that a specific biological process, not total calorie intake, controls the increase in lifespan.
Insulin/IGF
at glance: genetic pathways related to growth and insulin signaling are linked to aging
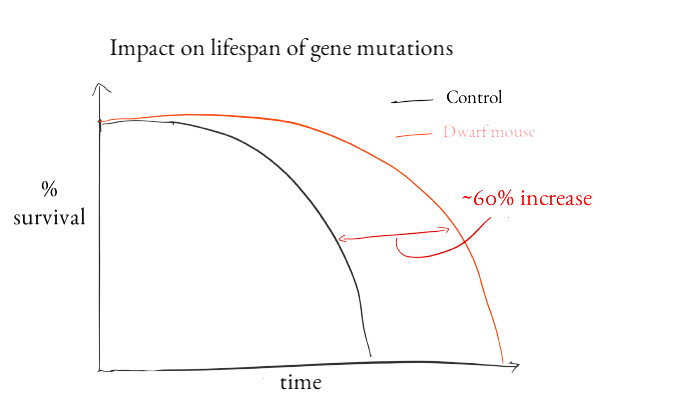
In papers published in 1983-1993, investigators introduced the concept that a gene could control lifespan. I got my start in science when one of the founders of this field, Cynthia Kenyon, agreed to let me work in her lab as a 12 year old kid. I'll always be grateful for her kindness and mentorship. Previously we'd known that caloric restriction could make animals live longer, but Kenyon and other scientists including Michael Klass, David Friedman and Tom Johnson found mutant genes that could make worms live longer. The gene that Kenyon found encoded a protein that is similar to insulin-like growth factor and insulin receptors in humans. In mice, mutating members of both of those pathways can increase lifespan. One of the longest-lived mouse mutants we have today is a dwarf mouse. In one study, people with similar dwarf mutations seemed to suffer less age-related disease than their non-mutated relatives.
I'm fascinated by the fact that many drugs which have been developed for diabetes, without any thought for their use elsewhere, turn out later to be relevant to aging. Good examples of this are metformin and FGF-21. Metformin is a small molecule used to treat Type 2 Diabetes, and FGF-21 is a protein in your blood that can increase lifespan in mice. We are still figuring out how the insulin/IGF pathway works, in particular what kinds of molecules might be driving the lifespan effect that we observe.
Parabiosis
at a glance: young blood makes old mice healthier, but why?
Dracula wanted to drink young blood, but what does that have to do with aging? A paper published in the 70's showed that linking old and young female mice so that they share a bloodstream increased lifespan. Decades later, in 2005, scientists at Stanford showed that this procedure might help old muscle stem cells repair wounds. Then, in 2011, a succession of papers came out showing that this procedure and others like it (such as injecting young blood into old mice) made mice better at remembering things, and improved heart and muscle function with age. These discoveries increased excitement and interest in the field, and lead to a wave of startups.
Investigators in the field have proposed many possible causes for this phenomenon. Proteins, small vesicles, or cells in the young mouse cleaning the blood of the old mouse might all be part of the effect. Many companies are trying to figure out whether there is a special protein or molecule involved. The big questions to resolve will be whether we can isolate a few key factors that are responsible for the parabiosis effect, and how many of the longevity-related phenotypes will translate to improve human health.
Senescence
at a glance: a fraction of your cells get older than the others, so we'd like to eliminate them
As you get old, so do your cells. But some of your cells get old in a way that is much worse than the others. You may have heard of a thing called telomerase. If you remember correctly, it's the thing that keeps the end of your DNA long enough that your cells can still divide. When one of your cells runs out of telomerase, it can't make many more copies of itself. If the cell sticks around, refuses to die even when it stops working, and starts secreting signals to the immune system, we call that a 'senescent cell'.
What happens when you get rid of these cells? Some animals that age faster than normal have a lot of these 'senescent cells' and are good experimental models in which to ask that question. In 2011, a group from the Mayo Clinic cleared out many of the senescent cells in one of those animal models, and found that the resulting mice were healthier in old age (among other things, they did not get cataracts and bent spines, which typically emerge in old age). In 2016, the same investigators found that getting rid of senescent cells in normal mice made them live a longer healthy lifespan. Knocking out senescent cells is tricky, because they don't have many unique identifiers. Companies are working to either find things empirically that kill senescent cells, or figure out specific mechanisms by which to try to destroy them.
Autophagy
at a glance: the garbage disposal unit of the cell worsens with age, improving it might increase healthy lifespan
Your body makes a lot of junk, on the molecular level, and cells need to clean this up. Just increasing the expression of one protein that helps to clean up this junk was enough to make mice live ~17% longer. Cells recycle old proteins and other molecules into a big vesicle, called a lysosome. It contains many proteins, and their job is to chop up old cell parts that it engulfs. Genes for proteins that do work in the lysosome are mutated in diseases such as Parkinson's. So improving this process has immediate relevance to neurodegenerative disease. As the lysosome gets older, more junk builds up in it that it cannot degrade. Finding ways to make more lysosomes, or help lysosomes degrade junk, may be interesting therapeutic avenues to pursue.
Hypothalamus
at a glance: a surprising number of things can increase lifespan when only changed in the brain tissue
Changing something just in the brain can be enough to make a mouse live longer. If the hypothalamus thinks it is too warm, for example, it can decrease the core body temperature of a mouse, resulting in a slightly longer lifespan. Changing the level of a variety of genes in a brain-specific way can also make a mouse live longer.We know that the hypothalamus makes something called growth hormone releasing hormone (GHRH), which is in charge of, well, releasing growth hormone. Growth hormone appears to be closely tied to lifespan, so the hypothalamus could be an important control point. One interesting question is how much you can affect the lifespan of a whole organism by just making changes to the brain.
Reproductive System
at a glance: removing the ability to reproduce can increase lifespan
10 years ago, one of the first projects I worked on was trying to understand a weird fact about reproduction in worms. If you take little worms and get rid of their gonads (I know, it's weird), they live ~60% longer than normal. But this only works if you get rid of the stuff inside (sperm/eggs - these worms are hermaphrodites, which means they carry around both). If you get rid of the whole thing, lifespan goes back to normal.
This isn't restricted to worms. From court records of Korean eunuchs, the eunuchs tend to live longer than their contemporaries by 14-19 years. Some people have tried to do things such as transplant young ovaries into old mice, to see if that helps (it might add a bit to lifespan). There are also many reports showing that when you make things live longer, fertility goes down. There might be a tradeoff (fertility takes away resources that could be used for something else), or a signal coming from the reproductive system that tries to hold up aging if it is damaged.
Mitochondria
at a glance: mitochondrial mutations impact lifespan in counterintuitive ways
You may have heard mitochondria referred to as the 'powerhouses' of the cell. It's funny, they do literally run like a dam generating hydroelectric power! - They pump protons (positively charged particles) one way, then use them as they slide back to run a kind of motor that makes a small energetic molecule used by many entities in the cell. One concept that comes up when people talk about mitochondria is 'oxidative stress' - the idea that if molecules are very reactive (say they have oxygen, acquire some extra electrons, and now want to discharge them onto other molecules), they are likely to interfere with a lot of other molecules in the cell that should be left to their own devices.
Weirdly, the story has turned on its head over time. It's true that it is bad to pump an animal full of reactive oxygen species, and that you can make a mouse live longer by increasing the level of proteins that are supposed to clean up mitochondria. But you can also mutate things that should be helping the mitochondria, and end up increasing lifespan! It's counterintuitive, and one hypothesis is that a little bit of stress is good because it forces your cells to put up their defenses and ramp up production of molecules that neuter the reactive oxygen species. But we don't really know.
Sirtuins
at a glance: sirtuins can change DNA and increase lifespan
Sirtuins add tags to the structural protein balls that DNA wraps around. It sounds odd, but think of yarn wrapping around a cardboard tube. When they add tags to the DNA yarn ball, it changes how the DNA is folded and expressed. So one of their actions is to control what genes do.
Sirtuins were first discovered to increase lifespan in yeast, and seem to also do so in worms, flies and mice. They depend on NAD to do their job, so when you see people talking about NR or other precursors of NAD, you can think about them as also helping the sirtuins do their job. You can extend lifespan a little bit in mice by giving them NR in old age.
[That's all for this part for now - I'm planning to add to the above, as time allows. There's lots more to talk about!]
Data on longevity
To give some context for the field, I thought it would be fun to do a more comprehensive survey. Below are virtually all the things we've seen that might improve mouse lifespan, listed in order of citations/year.
I think some of the papers on the bottom of the list are actually pretty cool and might have been given a short shrift
(Methodology - I took all papers that had a demonstrated mouse lifespan effect, extracted the key lifespan figure, and in cases where the text didn't list actual median or other lifespan used a virtual pixel ruler to count the pixels to the median point. Allow for some standard deviation of error accordingly!).
| Intervention | Median lifespan increase (treated/control) | Year Published | Notes | Reference |
|---|---|---|---|---|
| Senescent cell removal | 135% | 2016 | Does not affect rotarod performance, object discrimination. Slight delay in wound closure. | 1 |
| Rapamycin | 110% | 2009 | Late-life rapamyicn treatment extends lifespan (pooled females from multiple-site NIA study) | 2 |
| NR | 105% | 2016 | Claim an increase in running distance | 3 |
| Catalase | 117% | 2005 | Mitochondrially-targeted catalase expression extended mouse lifespan compared to control | 4 |
| Sirt6 overexpression | 115% | 2012 | Sirt6-overexpression increases male mouse lifespan | 5 |
For fun, I then took the list and matched the entries to drugs in the clinic. Part of the below might be outdated (companies sometimes take a long time to update on trial progress), but it's an interesting representation of the number of things in play that have a non-zero chance of having some impact on lifespan (would guess it to be pretty small in almost all cases though). Methodology is similar - just used clinicaltrials.gov to try to hunt down relevant trials and molecules, or googling generally.
| Longevity class | Drug | Phase | Developed For | Developer | Ref. |
|---|---|---|---|---|---|
| Insulin/IGF | |||||
| Growth Hormone Receptor Antagonist | |||||
| Pegvisomant (Somavert) | Approved (March 2003) | Acromegaly (normalizing IGF-1 levels) | Pfizer | 1 | |
| Akt1 antagonist | |||||
| Archexin (Akt1 antisense) | Phase 2 | Pancreatic/Renal Cancer | Rexahn Pharmaceuticals | 2 |
Thanks to Patrick Collison, Daniel Gross, Elad Gil and Nate Sauder for encouraging me to put this up, and Nathaniel Horwitz for major editorial help.
Appendix: What is a Kaplan-Meier curve?
Those curves you saw up top (illustrating metformin or eating less increasing lifespan) were generated by the following equation. It's ubiquitous in aging biology, and allows you to draw lifespan curves when, for example, some of the population could be censored.
d_i is the number of events at time i
n_i is the total individuals at risk at time i.
References
Table 1: 95 things that make mice live longer
- Baker, D. J., Childs, B. G., Durik, M., Wijers, M. E., Sieben, C. J., Zhong, J., … Deursen, J. M. Van. (2016). cells shorten healthy lifespan. Nature, 530(7589), 184–189. http://doi.org/10.1038/nature16932
- Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., … Miller, R. a. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392–5. doi:10.1038/nature08221
- Zhang, H., Ryu, D., Wu, Y., Gariani, K., Wang, X., Luan, P., … Auwerx, J. (2016). NAD repletion enhances life span in mice, 6(6292). http://doi.org/10.1126/science.aaf2693
- Schriner, S. E., Linford, N. J., Martin, G. M., Treuting, P., Ogburn, C. E., Emond, M., … Rabinovitch, P. S. (2005). Extension of murine life span by overexpression of catalase targeted to mitochondria. Science (New York, N.Y.), 308(5730), 1909–11. doi:10.1126/science.1106653
- Kanfi, Y., Naiman, S., Amir, G., Peshti, V., Zinman, G., Nahum, L., … Cohen, H. Y. (2012). The sirtuin SIRT6 regulates lifespan in male mice. Nature, 483(7388), 218–21. doi:10.1038/nature10815
- Martin-Montalvo, A., Mercken, E. M., Mitchell, S. J., Palacios, H. H., Mote, P. L., Scheibye-Knudsen, M., … de Cabo, R. (2013). Metformin improves healthspan and lifespan in mice. Nature Communications, 4. doi:10.1038/ncomms3192
- Zhang, G., Li, J., Purkayastha, S., Tang, Y., Zhang, H., Yin, Y., … Cai, D. (2013). Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature, 497(7448), 211–6. doi:10.1038/nature12143
- Kurosu, H., Yamamoto, M., Clark, J. D., Pastor, J. V, Gurnani, P., Mcguinness, O. P., … Kuro-o, M. (2008). Suppression of Aging in Mice by the Hormone Klotho, 309(5742), 1829–1833. http://doi.org/10.1126/science.1112766.Suppression
- Selman, C., Tullet, J. M. a, Wieser, D., Irvine, E., Lingard, S. J., Choudhury, A. I., … Withers, D. J. (2009). Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science (New York, N.Y.), 326(5949), 140–4. doi:10.1126/science.1177221
- Migliaccio, E., Giorgio, M., Mele, S., Pelicci, G., Reboldi, P., Pandolfi, P. P., … Pelicci, P. G. (1999). The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature, 402(6759), 309–13. doi:10.1038/46311
- Solon-Biet, S. M., McMahon, A. C., Ballard, J. W. O., Ruohonen, K., Wu, L. E., Cogger, V. C., … Simpson, S. J. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metabolism, 19(3), 418–430. http://doi.org/10.1016/j.cmet.2014.02.009
- Blüher, M., Kahn, B. B., & Kahn, C. R. (2003). Extended longevity in mice lacking the insulin receptor in adipose tissue. Science (New York, N.Y.), 299(5606), 572–4. doi:10.1126/science.1078223
- Latorre-Pellicer, A., Moreno-Loshuertos, R., Lechuga-Vieco, A. V., Sánchez-Cabo, F., Torroja, C., Acín-Pérez, R., … Enríquez, J. A. (2016). Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature, 535(7613), 561–565. http://doi.org/10.1038/nature18618
- Brandhorst, S., Choi, I. Y., Wei, M., Cheng, C. W., Sedrakyan, S., Navarrete, G., … Longo, V. D. (2015). A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metabolism, 22(1), 86–99. http://doi.org/10.1016/j.cmet.2015.05.012
- Miller, R. A., Harrison, D. E., Astle, C. M., Fernandez, E., Flurkey, K., Han, M., … Strong, R. (2014). Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell, 13(3), 468–477. http://doi.org/10.1111/acel.12194
- Satoh, A., Brace, C. S., Rensing, N., Cliften, P., Wozniak, D. F., Herzog, E. D., … Imai, S.-I. (2013). Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell metabolism, 18(3), 416–30. doi:10.1016/j.cmet.2013.07.013
- Mitchell, S. J., Martin-Montalvo, A., Mercken, E. M., Palacios, H. H., Ward, T. M., Abulwerdi, G., … De Cabo, R. (2014). The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Reports, 6(5), 836–843. http://doi.org/10.1016/j.celrep.2014.01.031
- Eisenberg, T., Abdellatif, M., Schroeder, S., Primessnig, U., Stekovic, S., Pendl, T., … Madeo, F. (2016). Cardioprotection and lifespan extension by the natural polyamine spermidine. Nature Medicine, 22(12), 1428–1438. http://doi.org/10.1038/nm.4222
- Pyo, J.-O., Yoo, S.-M., Ahn, H.-H., Nah, J., Hong, S.-H., Kam, T.-I., … Jung, Y.-K. (2013). Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nature communications, 4, 2300. doi:10.1038/ncomms3300
- Bernardes de Jesus, B., Vera, E., Schneeberger, K., Tejera, A. M., Ayuso, E., Bosch, F., & Blasco, M. a. (2012). Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Molecular Medicine, n/a–n/a. doi:10.1002/emmm.201200245
- Selman, C., Lingard, S., Choudhury, A. I., Batterham, R. L., Claret, M., Clements, M., … Withers, D. J. (2008). Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 22(3), 807–18. doi:10.1096/fj.07-9261com
- Flurkey, K., Papaconstantinou, J., Miller, R. a, & Harrison, D. E. (2001). Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proceedings of the National Academy of Sciences of the United States of America, 98(12), 6736–41. doi:10.1073/pnas.111158898
- Brown-Borg, H., Borg, K., Meliska, C., & Bartke, A. (1996). Dwarf mice and the aging process. Nature.
- Matheu, A., Maraver, A., Klatt, P., Flores, I., Garcia-Cao, I., Borras, C., … Serrano, M. (2007). Delayed ageing through damage protection by the Arf/p53 pathway. Nature, 448(7151), 375–9. http://doi.org/10.1038/nature05949
- Ozanne, S., & Hales, C. (2004). Lifespan: catch-up growth and obesity in male mice. Nature, 427(January).
- Miller, R. A., Buehner, G., Chang, Y., Harper, J. M., Sigler, R., & Smith-wheelock, M. (2005). Methionine-deficient diet extends mouse lifespan , slows immune and lens aging , alters glucose , T4 , IGF-I and insulin levels , and increases hepatocyte MIF levels and stress resistance, (February), 119–125. doi:10.1111/j.1474-9726.2005.00152.x
- Bitto, A., Ito, T. K., Pineda, V. V., Letexier, N. J., Huang, H. Z., Sutlief, E., … Kaeberlein, M. (2016). Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife, 5(AUGUST), 1–17. http://doi.org/10.7554/eLife.16351
- Coschigano, K. T., Clemmons, D., Bellush, L. L., & Kopchick, J. J. (2000). Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology, 141(7), 2608–13.
- Wu, J. J., Liu, J., Chen, E. B., Wang, J. J., Cao, L., Narayan, N., … Finkel, T. (2013). Increased Mammalian Lifespan and a Segmental and Tissue-Specific Slowing of Aging after Genetic Reduction of mTOR Expression. Cell reports, 4(5), 913–20. doi:10.1016/j.celrep.2013.07.030
- Ortega-Molina, A., Efeyan, A., Lopez-Guadamillas, E., Muñoz-Martin, M., Gómez-López, G., Cañamero, M., … Serrano, M. (2012). Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell metabolism, 15(3), 382–94. doi:10.1016/j.cmet.2012.02.001
- Hofmann, J. W., Zhao, X., De Cecco, M., Peterson, A. L., Pagliaroli, L., Manivannan, J., … Sedivy, J. M. (2015). Reduced expression of MYC increases longevity and enhances healthspan. Cell, 160(3), 477–488. http://doi.org/10.1016/j.cell.2014.12.016
- Zhang, Y., Xie, Y., Berglund, E. D., Coate, K. C., He, T. T., Katafuchi, T., … Mangelsdorf, D. J. (2012). The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife, 1, e00065. doi:10.7554/eLife.00065
- Baker, D. J., Dawlaty, M. M., Wijshake, T., Jeganathan, K. B., Malureanu, L., van Ree, J. H., … van Deursen, J. M. (2012). Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nature Cell Biology, 15(1), 96–102. http://doi.org/10.1038/ncb2643
- Yan, L., Vatner, D. E., O’Connor, J. P., Ivessa, A., Ge, H., Chen, W., … Vatner, S. F. (2007). Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell, 130(2), 247–58. doi:10.1016/j.cell.2007.05.038
- Harrison, D. E., Strong, R., Allison, D. B., Ames, B. N., Astle, C. M., Atamna, H., … Miller, R. a. (2013). Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell, (October), n/a–n/a. doi:10.1111/acel.12170
- Harrison, D. E., Strong, R., Allison, D. B., Ames, B. N., Astle, C. M., Atamna, H., … Miller, R. a. (2013). Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell, (October), n/a–n/a. doi:10.1111/acel.12170
- Riera, E., Huising, M. O., Follett, P., Leblanc, M., Halloran, J., & Andel, R. Van. (2014). TRPV1 Pain Receptors Regulate Longevity and Metabolism by Neuropeptide Signaling, 1023–1036. http://doi.org/10.1016/j.cell.2014.03.051
- Mercken, E. M., Mitchell, S. J., Martin-Montalvo, A., Minor, R. K., Almeida, M., Gomes, A. P., … de Cabo, R. (2014). SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell, 13(5), 787–796. http://doi.org/10.1111/acel.12220
- Satoh, S., Series, R., Forbush, B., Mcgloin, M., Tyryshkin, a M., Dismukes, G. C., … Walker, L. M. (2006). Transgenic Mice with a Reduced Core Body Temperature Have an Increased Life Span. Nature, 314(November), 825–828. http://doi.org/10.1126/science.1132191
- Nóbrega-Pereira, S., Fernandez-Marcos, P. J., Brioche, T., Gomez-Cabrera, M. C., Salvador-Pascual, A., Flores, J. M., … Serrano, M. (2016). G6PD protects from oxidative damage and improves healthspan in mice. Nature Communications, 7, 10894. http://doi.org/10.1038/ncomms10894
- Kappeler, L., De Magalhaes Filho, C., Dupont, J., Leneuve, P., Cervera, P., Périn, L., … Holzenberger, M. (2008). Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS biology, 6(10), e254. doi:10.1371/journal.pbio.0060254
- Dell’agnello, C., Leo, S., Agostino, A., Szabadkai, G., Tiveron, C., Zulian, A., … Zeviani, M. (2007). Increased longevity and refractoriness to Ca(2+)-dependent neurodegeneration in Surf1 knockout mice. Human molecular genetics, 16(4), 431–44. doi:10.1093/hmg/ddl477
- Sun, L., Sadighi Akha, A. A., Miller, R. A., & Harper, J. M. (2009). Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 64(7), 711–722. http://doi.org/10.1093/gerona/glp051
- Liu, X., Jiang, N., Hughes, B., Liu, X., Jiang, N., Hughes, B., … Hekimi, S. (2005). longevity : loss of mclk1 increases cellular fitness and lifespan in mice Evolutionary conservation of the clk-1 -dependent mechanism of longevity : loss of mclk1 increases cellular fitness and lifespan in mice, 2424–2434. doi:10.1101/gad.1352905
- Strong, R., Miller, R. a, Astle, C. M., Floyd, R. a, Flurkey, K., Hensley, K. L., … Harrison, D. E. (2008). Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging cell, 7(5), 641–50. doi:10.1111/j.1474-9726.2008.00414.x
- Strong, R., Miller, R. a, Astle, C. M., Floyd, R. a, Flurkey, K., Hensley, K. L., … Harrison, D. E. (2008). Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging cell, 7(5), 641–50. doi:10.1111/j.1474-9726.2008.00414.x
- Quick, K. L., Ali, S. S., Arch, R., Xiong, C., Wozniak, D., & Dugan, L. L. (2008). A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiology of aging, 29(1), 117–28. doi:10.1016/j.neurobiolaging.2006.09.014
- Muzumdar, R., Allison, D. B., Derek, M., Ma, X., Atzmon, G., Francine, H., … Barzilai, N. (2008). Visceral adipose tissue modulates mammalian longevity, 438–440. doi:10.1111/j.1474-9726.2008.00391.x
- Cai, W., He, J. C., Zhu, L., Chen, X., Wallenstein, S., Striker, G. E., & Vlassara, H. (2007). Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expression. The American journal of pathology, 170(6), 1893–902. doi:10.2353/ajpath.2007.061281
- Wang, J., Morita, Y., Han, B., Niemann, S., Löffler, B., & Rudolph, K. L. (2016). Per2 induction limits lymphoid-biased haematopoietic stem cells and lymphopoiesis in the context of DNA damage and ageing. Nature Cell Biology, 18(5), 480–490. http://doi.org/10.1038/ncb3342
- Anisimov, V., Popovich, I. G., Zabezhinski, M. A., Egormin, P. A., Yurova, M. N., Semenchenko, A. V., … Khaitsev, N. V. (2015). Sex differences in aging, life span and spontaneous tumorigenesis in 129/Sv mice neonatally exposed to metformin http://www.tandfonline.com/doi/pdf/10.4161/15384101.2014.973308. Cell Cycle, 14(1), 46–55. http://doi.org/10.4161/15384101.2014.973308
- Sun, L. Y., Spong, a., Swindell, W. R., Fang, Y., Hill, C., Huber, J. a., … Bartke, a. (2013). Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. eLife, 2, e01098–e01098. doi:10.7554/eLife.01098
- Hu, D., Cao, P., Thiels, E., Chu, C. T., Wu, G.-Y., Oury, T. D., & Klann, E. (2007). Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiology of learning and memory, 87(3), 372–84. doi:10.1016/j.nlm.2006.10.003
- Yang, X., Doser, T. A., Fang, C. X., Nunn, J. M., Janardhanan, R., Zhu, M., … Ren, J. (2006). Metallothionein prolongs survival and antagonizes senescence-associated cardiomyocyte diastolic dysfunction : role of oxidative stress. FASEB. doi:10.1096/fj.05
- Xu, J., Gontier, G., Chaker, Z., Lacube, P., Dupont, J., & Holzenberger, M. (2014). Longevity effect of IGF-1R+/- mutation depends on genetic background-specific receptor activation. Aging Cell, 13(1), 19–28. http://doi.org/10.1111/acel.12145
- Matheu, A., Maraver, A., Collado, M., Garcia-Cao, I., Cañamero, M., Borras, C., … Serrano, M. (2009). Anti-aging activity of the Ink4/Arf locus. Aging Cell, 8(2), 152–161. http://doi.org/10.1111/j.1474-9726.2009.00458.x
- Junnila, R. K., Duran-Ortiz, S., Suer, O., Sustarsic, E. G., Berryman, D. E., List, E. O., & Kopchick, J. J. (2016). Disruption of the GH receptor gene in adult mice increases maximal lifespan in females. Endocrinology, 157(12), 4502–4513. http://doi.org/10.1210/en.2016-1649
- Cargill, S. L., Carey, J. R., Müller, H.-G., & Anderson, G. (2003). Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging cell, 2(3), 185–90.
- Gates, A. C., Bernal-Mizrachi, C., Chinault, S. L., Feng, C., Schneider, J. G., Coleman, T., … Semenkovich, C. F. (2007). Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell metabolism, 6(6), 497–505. doi:10.1016/j.cmet.2007.10.010)
- Conover, C. a, Bale, L. K., Mader, J. R., Mason, M. a, Keenan, K. P., & Marler, R. J. (2010). Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. The journals of gerontology. Series A, Biological sciences and medical sciences, 65(6), 590–9. doi:10.1093/gerona/glq032
- López-Domínguez, J. A., Ramsey, J. J., Tran, D., Imai, D. M., Koehne, A., Laing, S. T., … McDonald, R. B. (2015). The Influence of Dietary Fat Source on Life Span in Calorie Restricted Mice. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 70(10), 1181–1188. http://doi.org/10.1093/gerona/glu177
- Enns, L. C., Morton, J. F., Treuting, P. R., Emond, M. J., Wolf, N. S., Dai, D.-F., … Ladiges, W. C. (2009). Disruption of protein kinase A in mice enhances healthy aging. PloS one, 4(6), e5963. doi:10.1371/journal.pone.0005963
- Mendias, C. L., Bakhurin, K. I., Gumucio, J. P., Shallal-Ayzin, M. V., Davis, C. S., & Faulkner, J. A. (2015). Haploinsufficiency of myostatin protects against aging-related declines in muscle function and enhances the longevity of mice. Aging Cell, 14(4), 704–706. http://doi.org/10.1111/acel.12339
- Nojima, A., Yamashita, M., Yoshida, Y., Shimizu, I., Ichimiya, H., Kamimura, N., … Minamino, T. (2013). Haploinsufficiency of akt1 prolongs the lifespan of mice. PloS one, 8(7), e69178. doi:10.1371/journal.pone.0069178
- Du, W. W., Yang, W., Fang, L., Xuan, J., Li, H., Khorshidi, A., … Yang, B. B. (2014). miR-17 extends mouse lifespan by inhibiting senescence signaling mediated by MKP7. Cell Death and Disease, 5(7), e1355. http://doi.org/10.1038/cddis.2014.305
- Spindler, S. R., Mote, P. L., Lublin, A. L., Flegal, J. M., Dhahbi, J. M., & Li, R. (2015). Nordihydroguaiaretic acid extends the lifespan of drosophila and mice, increases mortality-related tumors and hemorrhagic diathesis, and alters energy homeostasis in mice. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 70(12), 1479–1489. http://doi.org/10.1093/gerona/glu190
- Canaan, A., DeFuria, J., Perelman, E., Schultz, V., Seay, M., Tuck, D., … Weissman, S. M. (2014). Extended lifespan and reduced adiposity in mice lacking the FAT10 gene. Proceedings of the National Academy of Sciences, 111(14), 5313–5318. http://doi.org/10.1073/pnas.1323426111
- Bobkova, N. V., Evgen’ev, M., Garbuz, D. G., Kulikov, A. M., Morozov, A., Samokhin, A., … Nudler, E. (2015). Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proceedings of the National Academy of Sciences, 112(52), 16006–16011. http://doi.org/10.1073/pnas.1516131112
- Borrás, C., Monleón, D., Grueso, R. L., Gambini, J., Orlando, L., Pallardó, F. V, … Mora, J. F. De. (2011). RasGrf1 deficiency delays dging i n mice, 3(3), 262–276.
- Lopez-Mejia, I. C., De Toledo, M., Chavey, C., Lapasset, L., Cavelier, P., Lopez-Herrera, C., … Tazi, J. (2014). Antagonistic functions of LMNA isoforms in energy expenditure and lifespan. EMBO Reports, 15(5), 529–539. http://doi.org/10.1002/embr.201338126
- Wu, C.-Y., Chen, Y.-F., Wang, C.-H., Kao, C.-H., Zhuang, H.-W., Chen, C.-C., … Tsai, T.-F. (2012). A persistent level of Cisd2 extends healthy lifespan and delays aging in mice. Human molecular genetics, 21(18), 3956–68. doi:10.1093/hmg/dds210
- Spindler, S. R., Mote, P. L., Li, R., Dhahbi, J. M., Yamakawa, A., Flegal, J. M., … Lublin, A. L. (2013). β 1-Adrenergic receptor blockade extends the life span of Drosophila and long-lived mice. doi:10.1007/s11357-012-9498-3
- Spindler, S. R., Mote, P. L., Li, R., Dhahbi, J. M., Yamakawa, A., Flegal, J. M., … Lublin, A. L. (2013). β 1-Adrenergic receptor blockade extends the life span of Drosophila and long-lived mice. doi:10.1007/s11357-012-9498-3
- Miskin, R., & Masos, T. (1997). Transgenic mice overexpressing urokinase-type plasminogen activator in the brain exhibit reduced food consumption, body weight and size, and increased longevity. The Journal of Gerontology: Biological Science, 52(2), B118–B124. http://doi.org/10.1093/gerona/52A.2.B118
- Harper, J. M., Wilkinson, J. E., & Miller, R. a. (2010). Macrophage migration inhibitory factor-knockout mice are long lived and respond to caloric restriction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 24(7), 2436–42. doi:10.1096/fj.09-152223
- Singh, S. P., Niemczyk, M., Saini, D., Sadovov, V., Zimniak, L., & Zimniak, P. (2010). Disruption of the mgsta4 gene increases life span of C57BL mice. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 65(1), 14–23. http://doi.org/10.1093/gerona/glp165
- List, E. O., Berryman, D. E., Ikeno, Y., Hubbard, G. B., Funk, K., Comisford, R., … Kopchick, J. J. (2015). Removal of growth hormone receptor (GHR) in muscle of male mice replicates some of the health benefits seen in global GHR-/-mice. Aging, 7(7), 500–512.
- Doze, V. A., Papay, R. S., Goldenstein, B. L., Gupta, M. K., Collette, K. M., Nelson, B. W., … Perez, D. M. (2011). Long-Term ␣ 1A -Adrenergic Receptor Stimulation Improves Synaptic Plasticity , Cognitive Function , Mood , and Longevity, 80(4), 747–758. doi:10.1124/mol.111.073734.Norepinephrine
- Wu, S., Li, Q., Du, M., Li, S.-Y., & Ren, J. (2007). Cardiac-specific overexpression of catalase prolongs lifespan and attenuates ageing-induced cardiomyocyte contractile dysfunction and protein damage. Clinical and experimental pharmacology & physiology, 34(1-2), 81–7. doi:10.1111/j.1440-1681.2007.04540.x
- Zhang, S. Q., Cai, W. J., Huang, J. H., Wu, B., Xia, S. J., Chen, X. L., … Shen, Z. Y. (2015). Icariin, a natural flavonol glycoside, extends healthspan in mice. Experimental Gerontology, 69, 226–235. http://doi.org/10.1016/j.exger.2015.06.020
- Takeda, T., & Tanabe, H. (2016). Lifespan and reproduction in brain-specific MIR-29-knockdown mouse. Biochemical and Biophysical Research Communications, 471(4), 454–458. http://doi.org/10.1016/j.bbrc.2016.02.055
- Kawahara, M., & Kono, T. (2010). Longevity in mice without a father. Human reproduction (Oxford, England), 25(2), 457–61. doi:10.1093/humrep/dep400
- Andersen, J. B., Li, X. L., Judge, C. S., Zhou, A., Jha, B. K., Shelby, S., … Hassel, B. A. (2007). Role of 2-5A-dependent RNase-L in senescence and longevity, (October 2006), 3081–3088. doi:10.1038/sj.onc.1210111
- De Luca, G., Ventura, I., Sanghez, V., Russo, M. T., Ajmone-Cat, M. A., Cacci, E., … Calamandrei, G. (2013). Prolonged lifespan with enhanced exploratory behavior in mice overexpressing the oxidized nucleoside triphosphatase hMTH1. Aging cell, 12(4), 695–705. doi:10.1111/acel.12094
- Streeper, R. S., Grueter, C. a, Salomonis, N., Cases, S., Levin, M. C., Koliwad, S. K., … Farese, R. V. (2012). Deficiency of the lipid synthesis enzyme, DGAT1, extends longevity in mice. Aging, 4(1), 13–27.
- Hoeflich, A., Reyer, A., Ohde, D., Schindler, N., Brenmoehl, J., Spitschak, M., … Wolf, E. (2016). Dissociation of somatic growth, time of sexual maturity, and life expectancy by overexpression of an RGD-deficient IGFBP-2 variant in female transgenic mice. Aging Cell, 15(1), 111–117. http://doi.org/10.1111/acel.12413
- Conover, C. A., Bale, L. K., & Marler, R. J. (2015). Pregnancy-associated plasma protein-A deficiency improves survival of mice on a high fat diet. Experimental Gerontology, 70, 131–134. http://doi.org/10.1016/j.exger.2015.08.007
- Takahashi, K., Noda, Y., Ohsawa, I., Shirasawa, T., & Takahashi, M. (2014). Extended lifespan, reduced body size and leg skeletal muscle mass, and decreased mitochondrial function in clk-1 transgenic mice. Experimental Gerontology, 58, 146–153. http://doi.org/10.1016/j.exger.2014.08.003
- Redmann, S. M., & Argyropoulos, G. (2006). AgRP-deficiency could lead to increased lifespan. Biochemical and Biophysical Research Communications, 351(4), 860–864. http://doi.org/10.1016/j.bbrc.2006.10.129
- Kovina, M. V, Zuev, V. a, Kagarlitskiy, G. O., & Khodarovich, Y. M. (2013). Effect on lifespan of high yield non-myeloablating transplantation of bone marrow from young to old mice. Frontiers in genetics, 4(August), 144. doi:10.3389/fgene.2013.00144
- Shytikov, D., Balva, O., Debonneuil, E., Glukhovskiy, P., & Pishel, I. (2014). Aged mice repeatedly injected with plasma from young mice: a survival study. BioResearch Open Access, 3(5), 226–32. http://doi.org/10.1089/biores.2014.0043
- Markovich, D., Ku, M.-C., & Muslim, D. (2011). Increased lifespan in hyposulfatemic NaS1 null mice. Experimental Gerontology.
- Vereczki, V., Mansour, J., Pour-Ghaz, I., Bodnar, I., Pinter, O., Zelena, D., … Chinopoulos, C. (2016). Cyclophilin D regulates lifespan and protein expression of aging markers in the brain of mice. Mitochondrion, 34, 115–126. http://doi.org/10.1016/j.mito.2017.03.003
- Bale, L. K., West, S. A., & Conover, C. A. (2017). Inducible knockdown of pregnancy-associated plasma protein-A gene expression in adult female mice extends life span. Aging Cell, 1–3. http://doi.org/10.1111/acel.12624
- Grieb, B. C., Boyd, K., Mitra, R., & Eischen, C. M. (2016). Haploinsufficiency of the Myc regulator Mtbp extends survival and delays tumor development in aging mice, 8(10), 590–602.
Table 2: 70 things in the clinic that might make people live longer
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021106s031lbl.pdf
- https://clinicaltrials.gov/ct2/show/NCT02089334
- Chen, T., Hogan, S., Conley, G., Pazmany, C., Wu, Q.-L., McNeil, G. L., … Sexton, D. J. (2007). Discovery and characterization of human antibody inhibitors of pregnancy-associated plasma protein-A. Biological chemistry, 388(5), 507–12. doi:10.1515/BC.2007.058, Mikkelsen, J. H., Gyrup, C., Kristensen, P., Overgaard, M. T., Poulsen, C. B., Laursen, L. S., & Oxvig, C. (2008). Inhibition of the proteolytic activity of pregnancy-associated plasma protein-A by targeting substrate exosite binding. The Journal of biological chemistry, 283(24), 16772–80. doi:10.1074/jbc.M802429200
- Vikram, A., & Jena, G. (2010). S961, an insulin receptor antagonist causes hyperinsulinemia, insulin-resistance and depletion of energy stores in rats. Biochemical and biophysical research communications, 398(2), 260–5. doi:10.1016/j.bbrc.2010.06.070, Schäffer, L., Brand, C. L., Hansen, B. F., Ribel, U., Shaw, A. C., Slaaby, R., & Sturis, J. (2008). A novel high-affinity peptide antagonist to the insulin receptor. Biochemical and biophysical research communications, 376(2), 380–3. doi:10.1016/j.bbrc.2008.08.151
- https://clinicaltrials.gov/ct2/show/NCT03041701
- http://clinicaltrials.gov/ct2/show/NCT00791544
- http://clinicaltrials.gov/ct2/results?term=cixutumumab&Search=Search
- http://www.astellas.com/en/ir/library/pdf/2q2014_rd_en.pdf (Page 12), http://www.firstwordpharma.com/node/363806#axzz2o4na47BE
- http://clinicaltrials.gov/ct2/show/NCT01721577, http://www.axelar.se/docs/Axelar_PR13%20Results%20clinical%20study%20I-II%202011-09-26%20EN.pdf
- http://clinicaltrials.gov/ct2/show/NCT00673049, Ma, H., Zhang, T., Shen, H., Cao, H., & Du, J. (2013). The Adverse Events Profile of anti-IGF-1R Monoclonal Antibodies in Cancer Therapy. British journal of clinical pharmacology. doi:10.1111/bcp.12228
- http://clinicaltrials.gov/ct2/show/NCT01779336
- http://clinicaltrials.gov/ct2/results/displayOpt?flds=a&flds=b&flds=f&flds=n&flds=o&submit_fld_opt=on&term=biib022&show_flds=Y, http://meeting.ascopubs.org/cgi/content/abstract/28/15_suppl/2612
- http://clinicaltrials.gov/ct2/results/displayOpt?flds=a&flds=b&flds=f&flds=n&flds=o&submit_fld_opt=on&term=rg1507&show_flds=Y, Bagatell, R., Herzog, C. E., Trippett, T. M., Grippo, J. F., Cirrincione-Dall, G., Fox, E., … Gore, L. (2011). Pharmacokinetically guided phase 1 trial of the IGF-1 receptor antagonist RG1507 in children with recurrent or refractory solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research, 17(3), 611–9. doi:10.1158/1078-0432.CCR-10-1731
- http://clinicaltrials.gov/ct2/results?term=dalotuzumab&Search=Search, Atzori, F., Tabernero, J., Cervantes, A., Prudkin, L., Andreu, J., Rodríguez-Braun, E., … Baselga, J. (2011). A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research, 17(19), 6304–12. doi:10.1158/1078-0432.CCR-10-3336
- El-Ami, T., Moll, L., Carvalhal Marques, F., Volovik, Y., Reuveni, H., & Cohen, E. (2013). A novel inhibitor of the insulin/IGF signaling pathway protects from age-onset, neurodegeneration-linked proteotoxicity. Aging cell, 1–10. doi:10.1111/acel.12171
- Annunziata, M., Grande, C., Scarlatti, F., Deltetto, F., Delpiano, E., Camanni, M., … Granata, R. (2010). The growth hormone-releasing hormone (GHRH) antagonist JV-1-36 inhibits proliferation and survival of human ectopic endometriotic stromal cells (ESCs) and the T HESC cell line. Fertility and sterility, 94(3), 841–9. doi:10.1016/j.fertnstert.2009.03.093
- Siejka, A., Schally, A. V, Block, N. L., & Barabutis, N. (2010). Antagonists of growth hormone-releasing hormone inhibit the proliferation of human benign prostatic hyperplasia cells. The Prostate, 70(10), 1087–93. doi:10.1002/pros.21142
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/19667scm044_Sandostatin_lbl.pdf
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/200677lbl.pdf
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/019667s058,021008s023lbl.pdf
- http://clinicaltrials.gov/ct2/show/NCT00640757
- http://clinicaltrials.gov/ct2/show/NCT00427193
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021110s058lbl.pdf
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022088s002s004s005s007s010s012lbl.pdf
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022334s6lbl.pdf
- http://www.drugs.com/nda/ridaforolimus_120606.html, http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/oncologicdrugsadvisorycommittee/ucm296305.pdf
- Pearce, L. R., Alton, G. R., Richter, D. T., Kath, J. C., Lingardo, L., Chapman, J., … Alessi, D. R. (2010). Characterization of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1). The Biochemical journal, 431(2), 245–55. doi:10.1042/BJ20101024
- http://clinicaltrials.gov/ct2/show/NCT00937326, Venkatasubramanian, S., Noh, R. M., Daga, S., Langrish, J. P., Joshi, N. V, Mills, N. L., … Newby, D. E. (2013). Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. Journal of the American Heart Association, 2(3), e000042. doi:10.1161/JAHA.113.000042
- http://clinicaltrials.gov/ct2/show/NCT01416376
- http://clinicaltrials.gov/ct2/show/NCT00678015
- http://www.fda.gov/ohrms/dockets/ac/03/briefing/4012B1_03_Appd%201-Professional%20Labeling.pdf
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020357s031,021202s016lbl.pdf
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020482s024lbl.pdf
- https://clinicaltrials.gov/ct2/show/NCT02531334
- Ziegelbauer, K., Gantner, F., Lukacs, N. W., Berlin, A., Fuchikami, K., Niki, T., … Bacon, K. B. (2005). A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. British journal of pharmacology, 145(2), 178–92. doi:10.1038/sj.bjp.0706176
- Leung, C.-H., Chan, D. S.-H., Li, Y.-W., Fong, W.-F., & Ma, D.-L. (2013). Hit identification of IKKβ natural product inhibitor. BMC pharmacology & toxicology, 14(1), 3. doi:10.1186/2050-6511-14-3
- https://clinicaltrials.gov/ct2/show/NCT02439216
- Miller, S. C., Huang, R., Sakamuru, S., Shukla, S. J., Attene-, M. S., Shinn, P., … Austin, C. P. (2011). Signaling and their Mechanism of Action, 79(9), 1272–1280. doi:10.1016/j.bcp.2009.12.021.Identification
- http://clinicaltrials.gov/ct2/show/NCT01869959
- https://clinicaltrials.gov/ct2/show/NCT02097277?term=BMS-986036&rank=1
- http://clinicaltrials.gov/ct2/show/NCT01442584
- http://clinicaltrials.gov/ct2/show/NCT00545805
- http://www.vitaminshoppe.com/p/nature-plus-niacinamide-1000-mg-90-tablets/nt-1308#.UrsRAGRDulo
- https://chromadex.com/Ingredients/NIAGEN.html
- https://clinicaltrials.gov/ct2/show/NCT03176628
- http://www.amicusrx.com/preclinical.aspx
- https://clinicaltrials.gov/ct2/show/NCT01590888
- http://lysosomaltx.com
- Braeunig, J. H., Schweda, F., Han, P.-L., & Seifert, R. (2013). Similarly potent inhibition of adenylyl cyclase by P-site inhibitors in hearts from wild type and AC5 knockout mice. PloS one, 8(7), e68009. doi:10.1371/journal.pone.0068009
- http://clinicaltrials.gov/ct2/show/NCT01064492
- Murray, A. J. (2008). Pharmacological PKA inhibition: all may not be what it seems. Science signaling, 1(22), re4. doi:10.1126/scisignal.122re4
- http://clinicaltrials.gov/ct2/show/NCT01363141
- http://clinicaltrials.gov/ct2/show/NCT00739687
- http://clinicaltrials.gov/ct2/show/NCT00565318
- http://www.carolustherapeutics.com
- Al-Abed, Y., & VanPatten, S. (2011). MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future medicinal chemistry, 3(1), 45–63. doi:10.4155/fmc.10.281
- http://www.fda.gov/Drugs/DrugSafety/ucm225444.htm
- https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021846
- https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000032634
- https://www.nature.com/articles/nbt0104-3
- https://clinicaltrials.gov/ct2/show/NCT03268382
- https://clinicaltrials.gov/ct2/show/NCT02545283
- https://clinicaltrials.gov/ct2/show/NCT00779519
- https://clinicaltrials.gov/ct2/show/NCT02974153
- https://clinicaltrials.gov/ct2/show/NCT02959190
- https://clinicaltrials.gov/ct2/show/NCT02483585
- https://clinicaltrials.gov/ct2/show/NCT03303079
- https://clinicaltrials.gov/ct2/show/NCT02310763
- https://clinicaltrials.gov/ct2/show/NCT03039686
- http://www.cypralis.com/aims/research-portfolio
Per-section references for longevity explanations
Caloric Restriction
- McCay, C. M., Crowell, M. F., & Maynard, L. A. (1935). The effect of retarded growth upon the length of life span and upon the ultimate body size one figure. The journal of Nutrition, 10(1), 63-79.
- Solon-Biet, S. M., McMahon, A. C., Ballard, J. W. O., Ruohonen, K., Wu, L. E., Cogger, V. C., … Simpson, S. J. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metabolism, 19(3), 418–430. http://doi.org/10.1016/j.cmet.2014.02.009
- Miller, R. A., Buehner, G., Chang, Y., Harper, J. M., Sigler, R., & Smith-wheelock, M. (2005). Methionine-deficient diet extends mouse lifespan , slows immune and lens aging , alters glucose , T4 , IGF-I and insulin levels , and increases hepatocyte MIF levels and stress resistance, (February), 119–125. doi:10.1111/j.1474-9726.2005.00152.x
- Newman, J. C., Covarrubias, A. J., Zhao, M., Yu, X., Gut, P., Ng, C. P., ... & Verdin, E. (2017). Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell metabolism, 26(3), 547-557.
- Liao, C.-Y., Rikke, B. a, Johnson, T. E., Diaz, V., & Nelson, J. F. (2010). Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging cell, 9(1), 92–5. doi:10.1111/j.1474-9726.2009.00533.x
- Mattison, J. A., Colman, R. J., Beasley, T. M., Allison, D. B., Kemnitz, J. W., Roth, G. S., ... & Anderson, R. M. (2017). Caloric restriction improves health and survival of rhesus monkeys. Nature communications, 8.
- Wei, M., Brandhorst, S., Shelehchi, M., Mirzaei, H., Cheng, C. W., Budniak, J., ... & Cohen, P. (2017). Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Science translational medicine, 9(377), eaai8700.
Insulin/IGF
- Klass, M. (1983). A METHOD FOR THE ISOLATION OF LONGEVITY MUTANTS IN THE NEMATODE CAENORHABDITIS ELEGANS AND INITIAL RESULTS. Mechanisms of ageing and development, 22, 279–286.
- Friedman, D., & Johnson, T. (1987). A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics, 118(1), 75–86.
- Kenyon, C., Chang, J., Gensch, E., Rudner, A., & Tabtiang, R. (1993). A C. elegans mutant that lives twice as long as wild type. Nature.
- Blüher, M., Kahn, B. B., & Kahn, C. R. (2003). Extended longevity in mice lacking the insulin receptor in adipose tissue. Science (New York, N.Y.), 299(5606), 572–4. doi:10.1126/science.1078223
- List, E. O., Berryman, D. E., Ikeno, Y., Hubbard, G. B., Funk, K., Comisford, R., … Kopchick, J. J. (2015). Removal of growth hormone receptor (GHR) in muscle of male mice replicates some of the health benefits seen in global GHR-/-mice. Aging, 7(7), 500–512.
- Flurkey, K., Papaconstantinou, J., Miller, R. a, & Harrison, D. E. (2001). Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proceedings of the National Academy of Sciences of the United States of America, 98(12), 6736–41. doi:10.1073/pnas.111158898
- Brown-Borg, H., Borg, K., Meliska, C., & Bartke, A. (1996). Dwarf mice and the aging process. Nature.
- Guevara-Aguirre, J., Balasubramanian, P., Guevara-Aguirre, M., Wei, M., Madia, F., Cheng, C. W., ... & de Cabo, R. (2011). Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Science translational medicine, 3(70), 70ra13-70ra13.
- Martin-Montalvo, A., Mercken, E. M., Mitchell, S. J., Palacios, H. H., Mote, P. L., Scheibye-Knudsen, M., … de Cabo, R. (2013). Metformin improves healthspan and lifespan in mice. Nature Communications, 4. doi:10.1038/ncomms3192
- Zhang, Y., Xie, Y., Berglund, E. D., Coate, K. C., He, T. T., Katafuchi, T., … Mangelsdorf, D. J. (2012). The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife, 1, e00065. doi:10.7554/eLife.00065
Parabiosis
- Ludwig, F. C. and Elashoff, R. M. (1972), MORTALITY IN SYNGENEIC RAT PARABIONTS OF DIFFERENT CHRONOLOGICAL AGE*†. Transactions of the New York Academy of Sciences, 34: 582–587. doi:10.1111/j.2164-0947.1972.tb02712.x
- Conboy, I. M., Conboy, M. J., Wagers, A. J., Girma, E. R., Weissman, I. L., & Rando, T. a. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature, 433(7027), 760–4. http://doi.org/10.1038/nature03260
- Ruckh, J. M., Zhao, J. W., Shadrach, J. L., Van Wijngaarden, P., Rao, T. N., Wagers, A. J., & Franklin, R. J. M. (2012). Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell, 10(1), 96–103. http://doi.org/10.1016/j.stem.2011.11.019
- Villeda, S. a, Luo, J., Mosher, K. I., Zou, B., Britschgi, M., Bieri, G., … Wyss-Coray, T. (2011). The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature, 477(7362), 90–4. http://doi.org/10.1038/nature10357
- Smith, L. K., He, Y., Park, J.-S., Bieri, G., Snethlage, C. E., Lin, K., … Villeda, S. A. (2015). Β2-Microglobulin Is a Systemic Pro-Aging Factor That Impairs Cognitive Function and Neurogenesis. Nature Medicine, 21(8), 932–7. http://doi.org/10.1038/nm.3898
- Villeda, S. a, Plambeck, K. E., Middeldorp, J., Castellano, J. M., Mosher, K. I., Luo, J., … Wyss-Coray, T. (2014). Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nature Medicine, (May), 1–8. http://doi.org/10.1038/nm.3569
- Sinha, M., Jang, Y. C., Oh, J., Khong, D., Wu, E. Y., Manohar, R., … Wagers, A. J. (2014). Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science (New York, N.Y.), 344(6184), 649–52. http://doi.org/10.1126/science.1251152
- Loffredo, F. S., Steinhauser, M. L., Jay, S. M., Gannon, J., Pancoast, J. R., Yalamanchi, P., … Lee, R. T. (2013). Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell, 153(4), 828–839. http://doi.org/10.1016/j.cell.2013.04.015
- Castellano, J. M., Mosher, K. I., Abbey, R. J., McBride, A. A., James, M. L., Berdnik, D., … Wyss-Coray, T. (2017). Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature, 544(7651), 488–492. http://doi.org/10.1038/nature22067
Senescence
- Campisi, J. (2013). Aging, cellular senescence, and cancer. Annual review of physiology, 75, 685-705.
- Baker, D. J., Jeganathan, K. B., Cameron, J. D., Thompson, M., Juneja, S., Kopecka, A., ... & Van Deursen, J. M. (2004). BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nature genetics, 36(7), 744-749.
- Baker, D. J., Wijshake, T., Tchkonia, T., LeBrasseur, N. K., Childs, B. G., van de Sluis, B., … van Deursen, J. M. (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature, 479(7372), 232–6. doi:10.1038/nature10600
- Baker, D. J., Childs, B. G., Durik, M., Wijers, M. E., Sieben, C. J., Zhong, J., … Deursen, J. M. Van. (2016). cells shorten healthy lifespan. Nature, 530(7589), 184–189. http://doi.org/10.1038/nature16932
Autophagy
- Pyo, J.-O., Yoo, S.-M., Ahn, H.-H., Nah, J., Hong, S.-H., Kam, T.-I., … Jung, Y.-K. (2013). Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nature communications, 4, 2300. doi:10.1038/ncomms3300
- Sidransky, E., & Lopez, G. (2012). The link between the GBA gene and parkinsonism. The Lancet Neurology, 11(11), 986-998.
Hypothalamus
- Satoh, S., Series, R., Forbush, B., Mcgloin, M., Tyryshkin, a M., Dismukes, G. C., … Walker, L. M. (2006). Transgenic Mice with a Reduced Core Body Temperature Have an Increased Life Span. Nature, 314(November), 825–828. http://doi.org/10.1126/science.1132191
- Satoh, A., Brace, C. S., Rensing, N., Cliften, P., Wozniak, D. F., Herzog, E. D., … Imai, S.-I. (2013). Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell metabolism, 18(3), 416–30. doi:10.1016/j.cmet.2013.07.013
- Kappeler, L., De Magalhaes Filho, C., Dupont, J., Leneuve, P., Cervera, P., Périn, L., … Holzenberger, M. (2008). Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS biology, 6(10), e254. doi:10.1371/journal.pbio.0060254
- Miskin, R., & Masos, T. (1997). Transgenic mice overexpressing urokinase-type plasminogen activator in the brain exhibit reduced food consumption, body weight and size, and increased longevity. The Journal of Gerontology: Biological Science, 52(2), B118–B124. http://doi.org/10.1093/gerona/52A.2.B118
- Takeda, T., & Tanabe, H. (2016). Lifespan and reproduction in brain-specific MIR-29-knockdown mouse. Biochemical and Biophysical Research Communications, 471(4), 454–458. http://doi.org/10.1016/j.bbrc.2016.02.055
- Zhang, G., Li, J., Purkayastha, S., Tang, Y., Zhang, H., Yin, Y., … Cai, D. (2013). Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature, 497(7448), 211–6. doi:10.1038/nature12143
Reproductive System
- Hsin, H., & Kenyon, C. (2009). Signals from the reproductive system regulate the lifespan of C . elegans animal is extended . Our findings suggest that germline signals act, 399(April 1999).
- Yamawaki, T. M., Arantes-Oliveira, N., Berman, J. R., Zhang, P., & Kenyon, C. (2008). Distinct Activities of the Germline and Somatic Reproductive Tissues in the Regulation of Caenorhabditis elegans9 Longevity. Genetics, 178(1), 513-526.
- Min, K. J., Lee, C. K., & Park, H. N. (2012). The lifespan of Korean eunuchs. Current Biology, 22(18), R792-R793
- Cargill, S. L., Carey, J. R., Müller, H.-G., & Anderson, G. (2003). Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging cell, 2(3), 185–90.
Mitochondria
- Wu, S., Li, Q., Du, M., Li, S.-Y., & Ren, J. (2007). Cardiac-specific overexpression of catalase prolongs lifespan and attenuates ageing-induced cardiomyocyte contractile dysfunction and protein damage. Clinical and experimental pharmacology & physiology, 34(1-2), 81–7. doi:10.1111/j.1440-1681.2007.04540.x
- Schriner, S. E., Linford, N. J., Martin, G. M., Treuting, P., Ogburn, C. E., Emond, M., … Rabinovitch, P. S. (2005). Extension of murine life span by overexpression of catalase targeted to mitochondria. Science (New York, N.Y.), 308(5730), 1909–11. doi:10.1126/science.1106653
- Hu, D., Cao, P., Thiels, E., Chu, C. T., Wu, G.-Y., Oury, T. D., & Klann, E. (2007). Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiology of learning and memory, 87(3), 372–84. doi:10.1016/j.nlm.2006.10.003
- Quick, K. L., Ali, S. S., Arch, R., Xiong, C., Wozniak, D., & Dugan, L. L. (2008). A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiology of aging, 29(1), 117–28. doi:10.1016/j.neurobiolaging.2006.09.014
- Dell’agnello, C., Leo, S., Agostino, A., Szabadkai, G., Tiveron, C., Zulian, A., … Zeviani, M. (2007). Increased longevity and refractoriness to Ca(2+)-dependent neurodegeneration in Surf1 knockout mice. Human molecular genetics, 16(4), 431–44. doi:10.1093/hmg/ddl477
Sirtuins
- Kennedy, B. K., Austriaco, N. R., Zhang, J., & Guarente, L. (1995). Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell, 80(3), 485–96.
- Viswanathan, M., & Guarente, L. (2011). Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature, 477(7365), E1–E2. doi:10.1038/nature10440
- Rogina, B., & Helfand, S. L. (2004). Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proceedings of the National Academy of Sciences of the United States of America, 101(45), 15998–6003. doi:10.1073/pnas.0404184101
- Satoh, A., Brace, C. S., Rensing, N., Cliften, P., Wozniak, D. F., Herzog, E. D., … Imai, S.-I. (2013). Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell Metabolism, 18(3), 416–30. doi:10.1016/j.cmet.2013.07.013
- Kanfi, Y., Naiman, S., Amir, G., Peshti, V., Zinman, G., Nahum, L., … Cohen, H. Y. (2012). The sirtuin SIRT6 regulates lifespan in male mice. Nature, 483(7388), 218–21. doi:10.1038/nature10815
- Zhang, H., Ryu, D., Wu, Y., Gariani, K., Wang, X., Luan, P., … Auwerx, J. (2016). NAD repletion enhances life span in mice, 6(6292). http://doi.org/10.1126/science.aaf2693